
The National Institute for Health and Care Excellence (NICE) has published updated manuals for methods, processes and topic selection for Health Technology Assessments (HTA). This includes changes to how NICE evaluates treatments for patients with severe diseases. Pharmaceutical companies looking to achieve reimbursment in the severe disease space may now benefit from including a decision modifier (a value judgement previously applied to treatments at end of life) when calculating the value of their product for their HTA submission.
All evaluations starting after 1st February 2022 are subject to the new guidance. Exactly how these changes may affect you and your submission will vary – email info@mtechaccess.co.uk to discuss what this means for your health technology assessment strategy.
In light of this new guidance, we explore what a decision modifier is, when a product may qualify for a decision modifier for severity of disease, and gives an example of how decision modifiers are calculated.
What is a decision modifer?
Decision modifiers are factors or value judgements that are important to society and should be accounted for in decision making, but cannot be included in the cost-effectiveness calculation.
For example, prior to the release of the updated manuals, NICE committees could recommend treatments with a higher cost for people who were in the last months of their life, often referred to as the “end-of-life criteria”. Modifiers can be taken into account in decision making through qualitative discussion by the committee, or through quantitative weighting of quality-adjusted life years (QALYs) in the cost-effectiveness calculations.
During consultation for the updated manuals, NICE argued that there was no evidence that society valued end-of-life treatments over other therapies; instead, NICE noted evidence that society placed additional value on treatments that produced health gains for severe diseases. So, the end-of-life modifier was removed, and a decision modifier that applies extra weight to QALY gains for severe diseases was introduced.
Does my product qualify for a decision modifier for severity of disease?
NICE defines the severity of a disease or condition as the future health (including quality and length of life, i.e. QALYs) lost by people living with the disease or condition and receiving standard care in the NHS. Two estimates are considered in this context: the absolute QALY shortfall, and the proportional QALY shortfall. Both the absolute and the proportional QALY shortfall calculations should include discounting at 3.5%, in line with the NICE reference case.
1. The absolute QALY shortfall
The absolute QALY shortfall represents future QALYs lost from living with an illness and receiving the current standard of care when compared with what future QALYs would look like for someone without the illness. The absolute QALY shortfall is calculated by subtracting the future QALYs that people with the condition can expect, from the future QALYs that the general population with the same age and sex distribution can expect.
2. The proportional QALY shortfall
The proportional QALY shortfall is the proportion of future QALYs that are lost because of the condition. The proportional QALY shortfall is calculated by taking the absolute QALY shortfall and dividing it by the future QALYs that the general population with the same age and sex distribution would be expected to have.
The absolute or proportional QALY shortfall value will determine what weight is applied to the QALYs for your product in the evaluation, depending on whichever indicates the greatest severity. The weights are presented in the table below.
Table 1: QALY weights given proportional and absolute QALY shortfall
| QALY weight | Proportional QALY shortfall | Absolute QALY shortfall |
| 1 | Less than 0.85 | Less than 12 |
| 1.2 | 0.85 to 0.95 | 12 to 18 |
| 1.7 | At least 0.95 | At least 18 |
| Abbreviations: QALY, quality-adjusted life year. | ||
Decision modifier calculation for HTA – Worked example
Consider the following hypothetical disease:
- Patients with the disease who are treated with standard of care typically accrue 7 QALYs from the point of treatment to death
- Without the disease, the same person would be expected to accrue 20 future QALYs
- The person with the condition has fewer estimated future QALYs than those without the condition, and this is due to both reduced quality and quantity of life
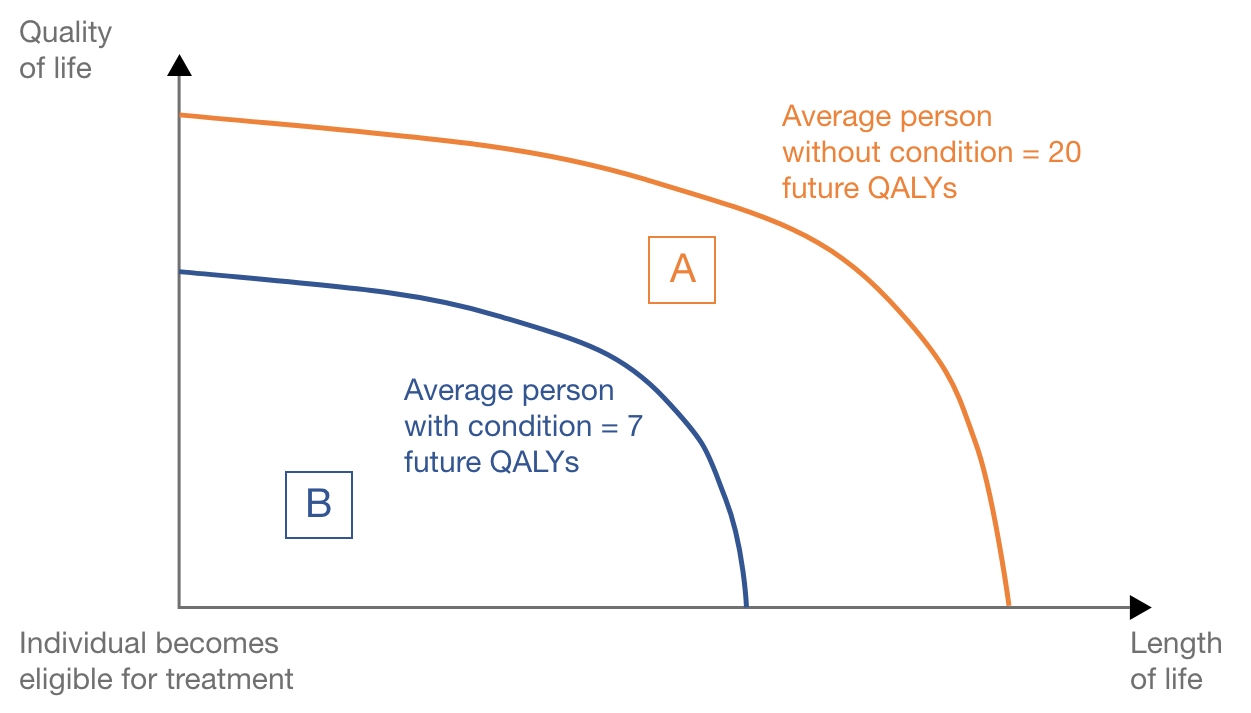
This hypothetical example is represented in Figure 1. The orange line represents the accumulation of QALYs for the average person without the disease, and the blue line represents an average person with the disease being treated with current standard of care.
The absolute QALY shortfall is equal to future QALYs without condition minus future QALYs with the condition = 20 – 7 = 13 QALYs, represented by area A in Figure 1. The absolute QALY shortfall is such that NICE would consider a QALY weight of 1.2 (QALY shortfall between 12 and 18).
The proportional QALY shortfall is equal to the absolute QALY shortfall divided by future QALYs without the condition = 13 / 20 = 0.65. Represented by area A divided by area A+B in Figure 1. The proportion of QALY shortfall is such that NICE would consider a QALY weight of 1 (proportional QALY shortfall less than 0.85).
Considering both the absolute and the proportional QALY shortfall, the weight that could be applied to the QALYs estimated within the cost-effectiveness analysis for your product is 1.2, the larger of the values for absolute and proportional QALY shortfall.
Figure 1. Graphical representation of a hypothetical disease
Abbreviations: QALY, quality-adjusted life year.
How can a decision modifier calculation impact your HTA submission?
So, what does this mean for your HTA submission? How can a decision modifier help your product reach patients?
Taking the example above, if this QALY weighting were included to support an HTA submission, it would increase the effective ICER threshold for the committee’s deliberations. Without a decision modifier, the effective threshold is generally considered to be in the range of £20,000 to £30,000 per additional QALY gained. With a multiplier of 1.2, this increases to an effective threshold of up to £36,000 per QALY gained. With a multiplier of 1.7, this increases further to an effective threshold of between £34,000 to £51,000, thereby increasing the likelihood of access to treatment for patients in England.
HTA Support from Mtech Access
If you would like to learn more about the HTA process, including the latest NICE updates and whether your product qualifies for a decision modifer, email info@mtechaccess.co.uk to explore how our experts can support your submission.


