
What is an Academy of Managed Care Pharmacy (AMCP) dossier and how are they used in US market access? Drawing on their extensive experience compiling AMCP dossiers, here, our global market access experts explain when, why and how AMCP dossiers are used, how they are formatted for formulary submissions, and how AMCP dossiers compare with global value dossiers.
Discover:
- What is an AMCP dossier?
- Who reads an AMCP dossier and why?
- When is an AMCP dossier used?
- How is an AMCP dossier formatted for formulary submissions?
- How does an AMCP dossier compare to a Global Value Dossier?
What is an AMCP dossier?
In the United States (US), the regulatory and legal environment governing communications between healthcare decision makers and drug and device manufacturers is complex. The Academy of Managed Care Pharmacy (AMCP) have developed a template to help Pharma standardise the way they present evidence for US-based healthcare decision makers. The AMCP dossier is widely accepted by different US stakeholders and decision-makers as the best-practice format for this information.
As such, the AMCP dossier offers a framework for drug and medical device manufacturers to provide clinical and economic evidence and other information to healthcare decision makers.
US healthcare decision makers use AMCP dossiers to evaluate a product for formulary, coverage, policy, or reimbursement within the US healthcare systems.
Who reads an AMCP dossier and why?
AMCP dossiers are not assessed by the AMCP in the way that European health technology assessment (HTA) bodies, like the National Institute for Health and Care Excellence (NICE) in the UK, assess HTA submissions. Instead, Pharma companies use AMCP dossiers to respond to validated and unsolicited requests for information from healthcare decision makers.
Ultimately, AMCP dossiers may be read by any US healthcare personnel, committee or organisation that uses an evidence-based process for making healthcare coverage and reimbursement decisions.
This might include payers, health plan managers, integrated delivery systems, pharmacy benefit management companies, specialty pharmacies, health insurance companies, medical groups, hospitals, hospital systems, Pharmacy and Therapeutic (P&T) Committees, health technology assessment (HTA) organisations, and other organised healthcare systems.
When is an AMCP dossier used?
The AMCP dossier can be used across the product lifecycle. Pre-launch and launch timed dossiers may have modelled projections based on clinical trial evidence and assumptions related to product uptake. Over time, real-world evidence can help inform formulary management and validate claims related to product value.
There are three types of dossiers that can be linked to different points in the development lifecycle:
- Unapproved product dossier: provides information about a product that is not currently approved by the US Food & Drug Administration (FDA). Manufacturers can use these dossiers to provide information to healthcare decision makers prior to FDA approval
- Approved product dossier: provides information about an FDA-approved product. Manufacturers may use this dossier to reactively provide information to a healthcare decision maker in response to an unsolicited request
- Unapproved use dossier: contains information about an approved product, but with a focus on a currently unapproved indication
Pharma and Medtech companies must have clear policies that dictate when dossiers are shared with healthcare decision makers. These policies should also address what constitutes an unsolicited request for a dossier and how to fulfil requests for information within the legal framework.
There is an organisational burden associated with the development and maintenance of an AMCP dossier. Market access teams must consider how to keep the dossier updated across the product life cycle.
For example, the dossier will need to be updated if there are changes to the prescribing information, line extensions, new safety information, or new information that affects the overall evidence.
Whilst this can be a lot of work, the AMCP dossier offers an effective and compliant way to provide payers with timely and trusted information about the latest clinical and economic evidence for a treatment.
How is an AMCP dossier formatted for formulary submissions?
Unlike a global value dossier, where there is flexibility to format the dossier to meet the needs of internal teams, an AMCP formulary dossier must follow a set template provided by the AMCP.
However, it is important that the needs and perspectives of the different US-based payer archetypes are kept front of mind during dossier development and updates. Take care to provide a clear value proposition and ensure your clinical and economic evidence meets the different needs of these different audiences. Your dossier should make the most of any communication opportunity.
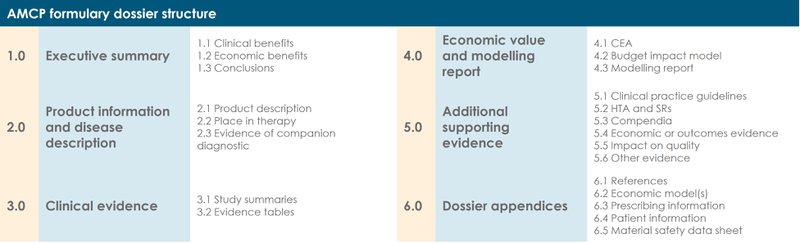
At the time of writing, the current AMCP format for Formulary Submissions (v4.1) contains 6 sections:
Abbreviations: AMCP, Academy of Managed Care Pharmacy; HTA, health technology assessment; SR, systematic review.
1.0 Executive summary
The executive summary should summarise the key clinical and economic value arguments outlined in sections 3.0–5.0. It should articulate the value argument to justify the expected expenditure in the context of the clinical evidence, health outcomes and economic consequences for the health system.
2.0 Product information and disease description
This section should provide a description of the product and a comparison with other products commonly used to treat the condition. It should also feature information on the disease, current approaches to treatment, and explain where your treatment would fit within the existing care pathway and/or how your treatment would change clinical practice.
3.0 Clinical evidence
The clinical evidence section is made up of study summaries and evidence tables. It should present all the clinical studies that support the value of the product. Where a product has more than one approved indication, this section can become quite long. We sometimes recommend to our clients that they supply separate dossiers for each approved indication.
4.0 Economic value and modelling report
The economic value and modelling section will typically include a cost-effectiveness analysis, budget impact model, and modelling report. The intent of the model(s) is to quantify the risk–benefit trade-off of the product and its economic value. Budget impact and cost-effectiveness models should be developed in line with best practice published by The Professional Society for Health Economics and Outcomes Research (ISPOR).
It is important to ensure your model is developed by an experienced health economist. Discover how our health economic modellers recently built a model for a client’s AMCP dossier in this case study.

5.0 Additional supporting evidence
This section includes any additional supporting evidence that healthcare decision makers should consider, that is not already included in the previous sections. This may include clinical practice guidelines, HTA reports and systematic reviews, compendia, and pharmacoeconomic studies.
6.0 Dossier appendices
The appendices should include references and any additional economic models, along with the FDA-approved label, package insert, patient package insert and the materials safety data sheet.
How does an AMCP dossier compare to a Global Value Dossier?
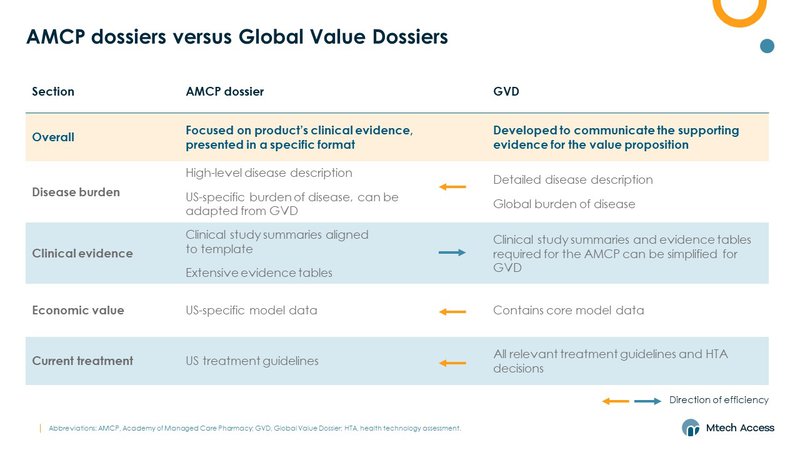
Whilst there are many similarities between an AMCP dossier and the internal global value dossier (GVD) many companies develop, there are some key differences around the level of detail and market specifics included.
Abbreviations: AMCP, Academy of Managed Care Pharmacy; GVD, global value dossier; HTA, health technology assessment.
We have found that there are efficiencies to be leveraged from developing a GVD and AMCP dossier in tandem.
Sections that require more detail in one dossier can be developed for that one and then streamlined for the other. For example, the detailed clinical section required for the AMCP may be developed first and simplified for the GVD. Whereas the detailed burden and current treatment sections typically included in a GVD can be developed first, as these often include a wide range of market data. These can then be streamlined for the ACMP dossiers, with the focus on US-specific data .
As such, it can be helpful to have the same global agency develop both.
(But then, as experts in dossier writing eager to support our clients with both their AMCP dossier and GVD projects, we would say that!)
For support developing an AMCP dossier or GVD, or for support with your US market access strategy, email info@mtechaccess.co.uk to book a meeting with our specialist teams.


