Calum Jones, Consultant Health Economist at Mtech Access, shares his passion for helping MedTech firms build health economic models that help their innovative new technologies reach patients.
Tell us about your role as a Consultant Health Economist at Mtech Access.
As a Consultant Health Economist with 8 years of experience, my role is to project manage, conceptualise, develop, and communicate the outputs of economic models designed to examine the value and/or financial impact of pre-launch pharmaceutical or MedTech assets.
Most of the health economic models that my colleagues and I develop are commissioned by pharmaceutical companies. However, there is a rapidly emerging evidence base and corresponding evidence thresholds for MedTech assets, meaning that health economic models are now more relevant than ever for MedTech companies.
Why is health economics increasingly relevant for MedTech?
Health economists strive to facilitate decision-making that leads to efficient use of scarce resources, typically weighing up the costs and benefits of two or more competing products. This goal is usually achieved through a regulator-recognised and approved modelling procedure.
Traditionally, health economic models have been required of big pharmaceutical companies by health technology assessment agencies. Increasingly, MedTech and digital health innovators face similar evidence generation needs.
The UK Department for Health and Social Care has recently updated1 its nascent code of conduct for data-driven healthcare technologies, detailing the types of evidence MedTech companies are expected to produce to gain adoption in the UK NHS.
I expect these developments to be broadly reflective of what we will begin to see in the next few years in countries with established pharmaceutical health technology assessment standards and processes.
How can a health economic model help a new medical technology reach patients?
A central tenet of the updated code of conduct is the encouragement of MedTech developers to create a clear value proposition for their assets.
All developers – be they MedTech or pharmaceutical – must determine early on what problem their asset is intended to address, who their asset will benefit, and how this is expected to be achieved.
All health outcome, efficiency, and financial benefits should be defined from the perspective of patients and health system providers and framed against the asset’s nearest comparator(s) (i.e. the existing competing solution to the defined problem).
Health economic modelling practices are ideally suited to guiding MedTech developers through this preliminary decision problem-defining process. This is achieved by characterising the relevant patient population, defining value drivers, mapping the patient and treatment pathway(s), and identifying expected relevant health benefits, (potential) harms, costs, and savings.
A second pillar of the updated code of conduct is for MedTech developers to generate evidence that their asset achieves clinical, social, economic, or behavioural benefits.
MedTech assets targeting national recommendation or reimbursement may be reviewed by the country’s relevant health technology assessment agency (e.g. NICE). The agency will likely examine both clinical effectiveness and economic impact, and perhaps the relative value of the asset. Regional submissions will likely also require at minimum some demonstration of acceptable budgetary impact for the budget holder in question.
The role of different health economic models is to allay target stakeholder concerns around the robustness of claims relating to efficacy, cost, or value. The models are underpinned by clinically and industry-appropriate structured base case estimates alongside deterministic and probabilistic uncertainty analyses.
What information and evidence do MedTech companies need to begin conceptualising a health economic model?
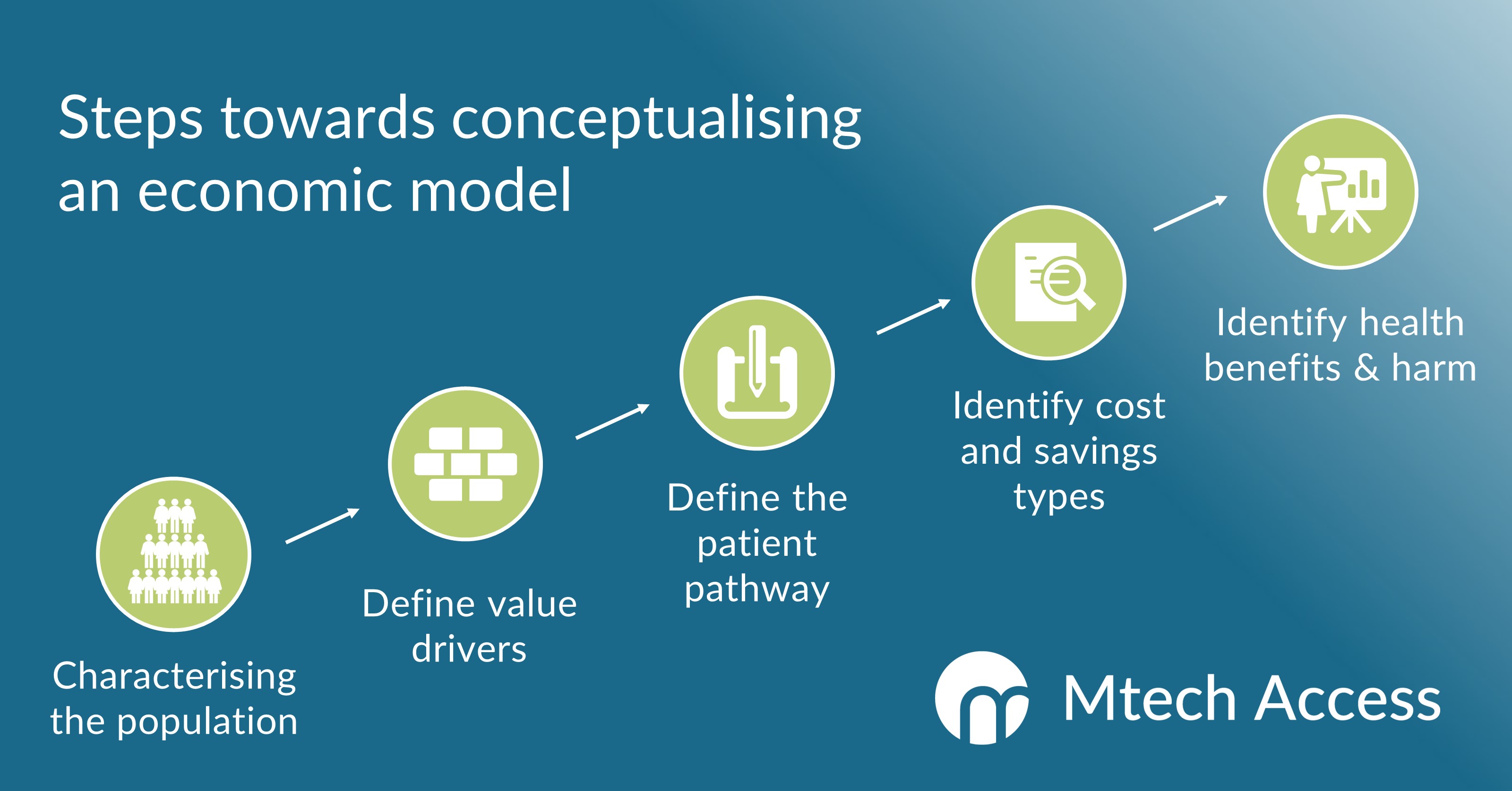
The conceptualisation of a health economic model should feature several key steps.
First, the decision problem and target patient population should be characterised.
The next step is to define the key value drivers of the asset, i.e., the clinical metrics that will underpin the demonstration of cost savings and/or relative value.
The mapping of the patient pathway is central to the conceptualisation process, conventionally defining a stepwise, multi-branch flow of states that a patient can occupy over time.
The process concludes with the identification and sourcing of clinical benefits and direct and/or indirect costs which can occur along the patient pathway.
The conceptualisation process will directly inform the appropriate type of model to develop and the approach to take.
Which health economic methodology/model would you typically recommend for a novel MedTech intervention?
Where the perceived need is for the developer to demonstrate the value of the new asset relative to the closest comparator (such as in the UK, France, Canada, and Australia), a form of cost-effectiveness model will be required.
A cost-effectiveness model estimates the ratio of the difference in costs associated with both the asset and comparator over a measure of benefit.
In some countries, a quality of life-based measure is the favoured unit of benefit, typically reflected in the use of quality-adjusted life years – a measure capturing both the quality and quantity of life lived.
In the UK, NICE may review a medical technology through the Medical Technologies Evaluation Programme (MTEP). In this instance, a cost-consequence analysis is required.2
Budget impact models are typically required at the regional and national level for most countries that have established health technology assessment bodies and processes. A budget impact model estimates the expected financial impact of introducing the new asset into the payer’s asset portfolio, accounting for key epidemiological, demographic, and market share assumptions. Such estimates are usually made over a 5-year time horizon.
What one piece of advice would you give a MedTech company launching a novel innovation?
Without doubt, my main advice for a MedTech company would be to start collecting and/or sourcing key evidence as soon as possible. Success in demonstrating the relative favourability of the new asset will depend upon the availability of high-quality data.
Companies will need a range of data, including direct cost and quality of life data, relevant for both the company’s asset and the selected comparator.
You mentioned earlier that you were particularly interested in how health economics can support MedTech innovations. What inspires you in this area?
Conceptualising and developing health economic models for MedTech companies has been a key focus of my professional time over the past 2 years.
The principle of maximising the benefit derived from scarce resources is central to the practice of health economics. New MedTech innovations offer the promise of unlocking benefits which likely could not be realised under more conventional means. For me, the increasing proliferation of such assets opens up a whole new world of intrigue and intellectual stimulation.
Working closely with notable UK-based MedTech innovation fellowship organisations, I’ve held numerous one-to-one workshops with new innovators to systematically co-run the required modelling conceptualisation process. For some of these innovators I’ve proceeded to develop tailored economic models for regional and national negotiations in key European markets.
My experience and interest in working closely with MedTech innovators combines well with the heritage and ongoing work of Mtech Access, which has worked with multiple MedTech companies since its inception in 2015, alongside its pharmaceutical client base.
To find out more about our approach to health economic modelling or to discuss how we can help your MedTech innovation reach patients, email info@mtechaccess.co.uk to arrange a no-obligation consultation meeting.
References:
- Department of Health and Social Care. A guide to good practice for digital and data-driven health technologies. 2021. Available from: https://www.gov.uk/government/publications/code-of-conduct-for-data-driven-health-and-care-technology/initial-code-of-conduct-for-data-driven-health-and-care-technology. Accessed on: 25th January 2021
- National Institute for Health and Care Excellence. Medical technologies evaluation programme methods guide. 2017. Available from: https://www.nice.org.uk/process/pmg33/resources/medical-technologies-evaluation-programme-methods-guide-pdf-72286774205893. Accessed on: 25th January 2021



[…] this webinar, Calum and Nick from our health economics team will explore why health economics is becoming a key part of market access for MedTech innovations and how health economic models can provide the evidence these innovations need to […]