Case Study
What our client needed
Our top 20 Pharma client was looking to build a gold standard evidence base to support their global regulatory and reimbursement strategy for the launch of a key oncology product.
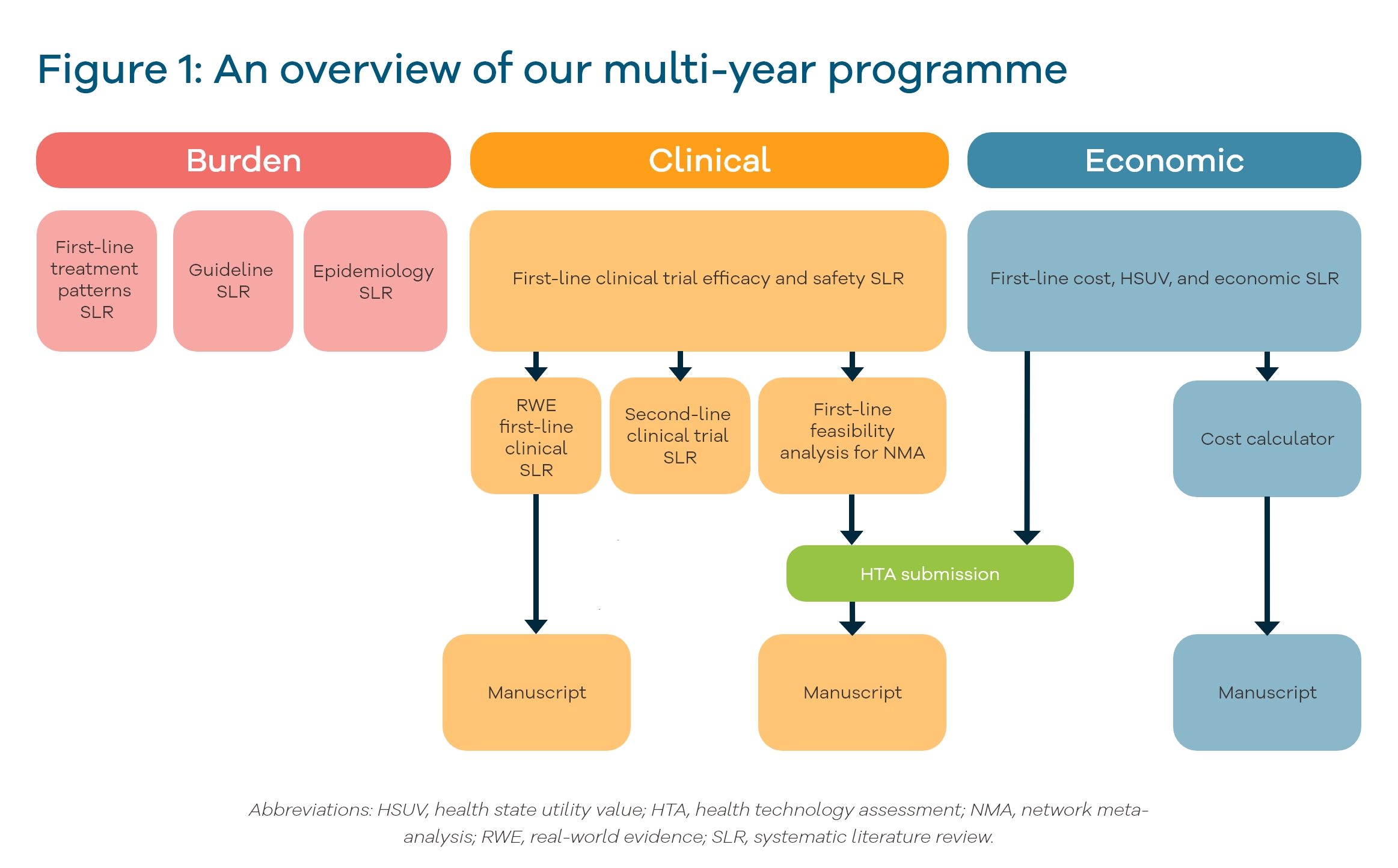
We were initially approached to run an early clinical systematic literature review. This has developed into a multi-year programme of work, covering real-world evidence, clinical, and economic systematic literature reviews. The client asked us to support with updates, new analyses, and manuscripts, to inform their reimbursement strategy in response to an evolving market landscape.
How we supported them
Our expert Evidence Team collaborated with our client to ensure that the evidence base was up to date and fit for purpose for each reimbursement milestone.
We started with systematic literature reviews of clinical trial efficacy and safety data for their product and competitor first-line treatments. We also conducted feasibility analyses to identify whether indirect comparisons of treatments would be appropriate in the absence of head-to-head trials. During our multi-year engagement with the client, the systematic literature reviews were updated several times to reflect new evidence relevant to upcoming health technology assessment (HTA) submissions. We also performed complementary reviews focused on patients with specific mutational profiles. These informed an indirect treatment comparison, which is soon to be reported in a journal publication.
We also conducted economic systematic literature reviews that assessed health state utility values and first-line treatment costs. These were updated in line with the clinical reviews.
Our Health Economics Team developed a cost calculator to estimate the financial impact of treatment-related adverse events, which was later incorporated into a manuscript.
The evidence base to support HTA submissions was developed further, with a review of real-world evidence, second-line treatments, treatment patterns, treatment guidelines, epidemiology, and burden of disease. The real-world evidence systematic literature review captured a large amount of data, and to help our client identify the most relevant information, we created an evidence map to summarise and visualise the different study characteristics and outcomes. The output of the real-world evidence systematic literature review was published as a journal article, illustrating that current treatments did not significantly improve patient survival and that innovative treatments were needed.
The outcome
Our client had a comprehensive, up-to-date, gold standard evidence base for each stage of their reimbursement and market access journey. This contributed to a successful National Institute for Health and Care Excellence (NICE) HTA, which was informed by the clinical and economic systematic literature reviews. We continue to expand and update the evidence that we generated, in order to support new market access strategies.
How we added value
Utilising their extensive existing, in-depth experience in the client’s specific area of oncology, our Evidence Team were able to rapidly develop an evidence strategy based on a broad understanding of the product landscape.
Our Evidence Team further streamlined the client journey by mapping the evidence to help unravel large, heterogeneous datasets. They provided engaging visual representations of data in the form of waterfall and forest plots, breaking down some of the more complex evidence into a digestible format.
Overall, we have established a long-term and highly collaborative relationship with our client, and continue to work with them as a trusted partner on the same drug and disease programme to provide a cutting-edge approach.