
Our global market access experts have put together this framework of activities or stepping stones for successful market access. Download the PDF summary here.
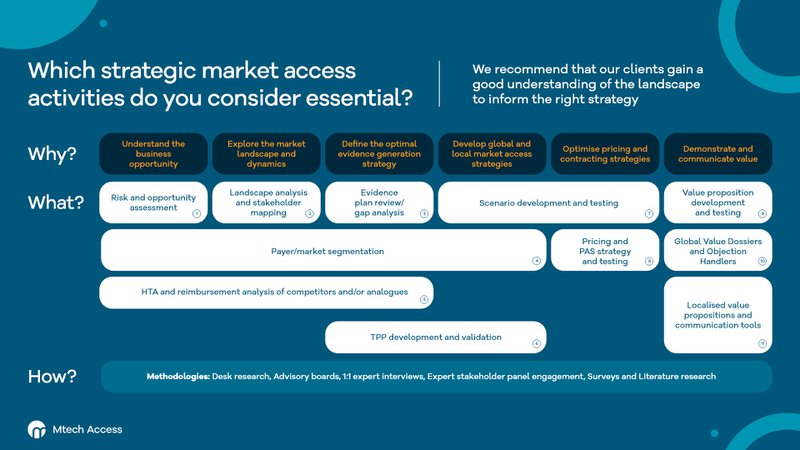
These activities are designed to help Pharma and MedTech teams at different stages of their market access journey. The framework includes activities for those looking to:
- Understand the business opportunity
- Explore the market landscape and dynamics
- Define the optimal evidence generation strategy
- Develop global and local market access strategies
- Optimise pricing and contracting strategies
- Demonstrate and communicate value
Strategic market access activities
The framework describes 11 activities that our specialist market access and HEOR teams can conduct to support Pharma and MedTech market access teams.

1) Risk and opportunity assessment
Challenge: To identify commercial potential of a new product in development or acquisition and inform positioning
Solutions: Review disease landscape, patient journey, current standard of care, current and future competitors
2) Landscape analysis and stakeholder mapping
Challenge: To identify challenges and opportunities and plan accordingly
Solutions: Review current and future landscape (e.g. reimbursement stakeholders, decision-making processes and evidence requirements, future environmental and policy changes)
3) Evidence plan review/gap analysis
Challenge: To highlight gaps and plan to mitigate
Solutions: Review planned and available evidence against payer requirements
4) Payer/market segmentation
Challenge: To understand reimbursement potential and plan launch sequence
Solutions: Review market and payer landscape
5) HTA and reimbursement analysis of competitors and/or analogues
Challenge: To identify the optimal supporting evidence development strategy, including trial design, choice of endpoint and outcome measure(s), identify gaps or areas of data weakness so these can be mitigated
Solutions: Review past HTA decisions for competitors and/or analogues
6) TPP development and validation
Challenge: To gain insights on the TPP to optimise market access strategy and communication
Solutions: Primary payer research (interviews, survey, advisory board)
7) Scenario development and testing
Challenge: To refine and optimise strategy
Solutions: Generate reimbursement and access scenarios to test with internal and external experts
8) Pricing and PAS strategy and testing
Challenge: To gain insights to optimise pricing strategy in key markets
Solutions: Primary payer research (interviews, survey, advisory board)
9) Value proposition development and testing
Challenge: To gain insights to optimise and convey the value proposition to relevant stakeholders in key markets
Solutions: Primary payer research (interviews, survey, advisory board)
10) Global value dossiers and objection handlers
Challenge: To ensure consistent internal communication and inform external communications and reimbursement negotiations
Solutions: Materials to communicate product value
11) Localised value propositions and communication tools
Challenge: To optimise external communications and local reimbursement negotiations
Solutions: Digital tools to communicate product value
Local experts inform these strategic market access activities
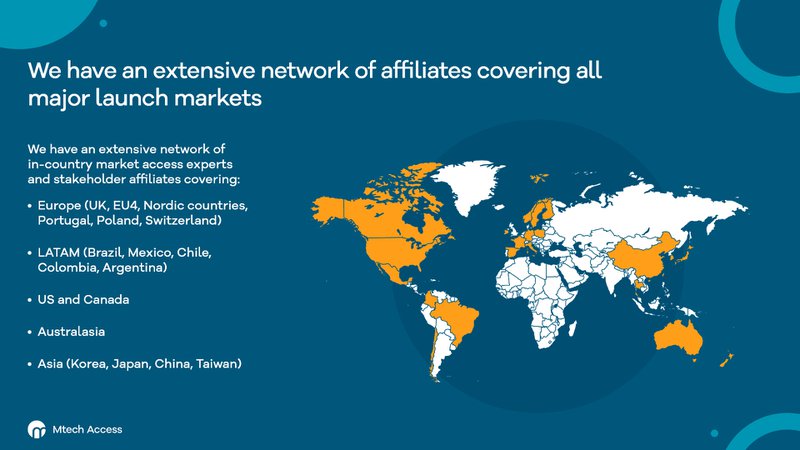
We have an extensive network of in-country market access experts and stakeholder affiliates covering:
- Europe (UK, EU4, Nordic countries, Portugal, Poland, Switzerland)
- LATAM (Brazil, Mexico, Chile, Colombia, Argentina)
- US and Canada
- Australasia
- Asia (Korea, Japan, China, Taiwan)
We consult the relevant local experts to ensure each activity, and your overall strategy, are aligned with the requirements and nuances of all your key local markets.
The activities essential for your market access journey will vary for different products and markets. Relevant activities will also depend on the lifecycle stage of the product and what you are looking to achieve. Contact our team today to learn more about the framework and to discuss your specific needs with our experts.


